Projects
Modulating T Cell Alloimmunity in Transplantation
Our lab investigates the molecular pathways that regulate T cell responses in transplantation. We explore how immune signaling influences alloreactivity, graft rejection, and tolerance. By studying key immune regulators, we aim to develop novel strategies to suppress harmful immune responses while preserving protective regulatory mechanisms. Our work provides insights into immune modulation and lays the foundation for innovative therapies to improve transplant outcomes.
Uehara, M. et al. Regulation of T cell alloimmunity by PI3Kγ and PI3Kδ. Nat Commun 8, 951 (2017). https://doi.org/10.1038/s41467-017-00982-xLymph Node Stromal Cells in Immune Regulation
We study how lymph node stromal cells influence immune activation and tolerance. Our research focuses on fibroblastic reticular cells (FRCs) and high endothelial venules (HEVs) as key regulators of immune responses. By understanding how these structures shape T cell activation, migration, and immune suppression, we aim to develop targeted therapies that enhance immune tolerance in transplantation and autoimmunity.
Zhao, J. et al. Delivery of costimulatory blockade to lymph nodes promotes transplant acceptance in mice. Journal of Clinical Investigation 132, e159672 (2022). https://www.jci.org/articles/view/159672Harnessing Immune Mechanisms to Improve Cancer Therapy
We explore immune-based strategies to enhance cancer treatment by combining direct tumor targeting with immune system activation. Our research focuses on overcoming immune suppression in the tumor microenvironment and identifying molecular targets that enable both tumor destruction and long-lasting antitumor immunity. By integrating immunotherapy with novel molecular approaches and nano-targeted delivery, we aim to develop more effective and durable cancer treatments.
Jiang, L. et al. Direct Tumor Killing and Immunotherapy through Anti-SerpinB9 Therapy. Cell 183, 1219-1233.e18 (2020). https://doi.org/10.1016/j.cell.2020.10.045Targeted Immune Therapy for Autoimmune Disease
Our lab develops innovative immune therapies to restore immune balance in autoimmune diseases. We focus on targeted drug delivery systems that enhance the precision of immunotherapies while minimizing side effects. By leveraging lymph node-specific nanoparticle technology, we aim to improve the efficacy of immune interventions and provide new treatment strategies for conditions such as type 1 diabetes.
Jung, S. et al. Nanotargeted Delivery of Immune Therapeutics in Type 1 Diabetes. Advanced Materials 35, 2300812 (2023). https://doi.org/10.1002/adma.202300812Lymph Node Stromal Cells in Organ Injury and Repair
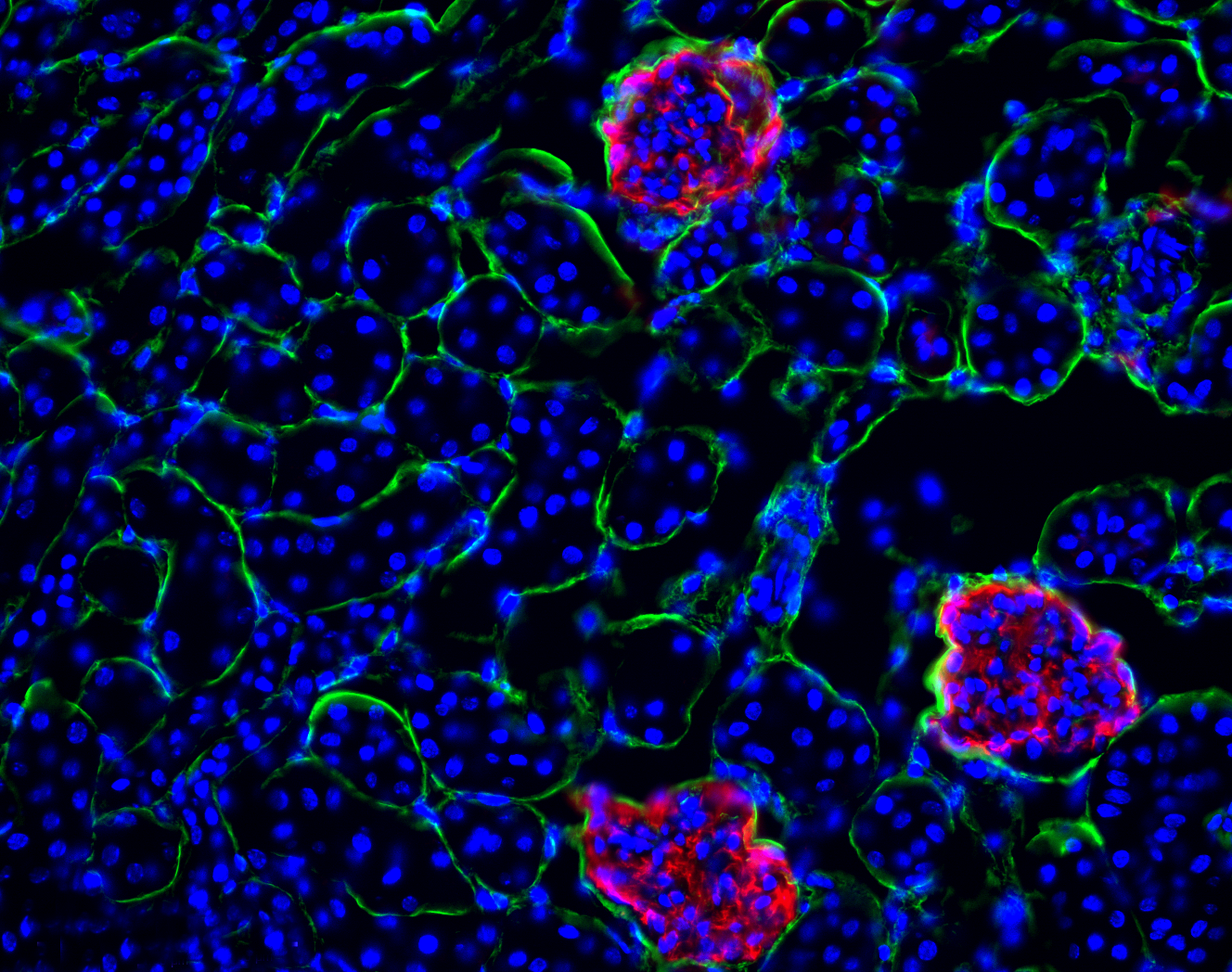
Our lab explores how immune-stromal interactions influence organ damage and recovery. We investigate the role of lymph node fibroblastic reticular cells (FRCs) and high endothelial venules (HEVs) in shaping immune responses following injury. Our research demonstrates that lymph node remodeling can drive both immune activation and tissue fibrosis, while targeted modulation of stromal cells may promote repair. By studying these mechanisms in the context of ischemia-reperfusion injury, we aim to uncover novel therapeutic strategies for mitigating inflammation and fibrosis across different organ systems.
Maarouf, O. H. et al. Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing. JCI Insight 3, e120546 (2018). https://doi.org/10.1172/jci.insight.120546Targeted Drug Delivery to Lymph Nodes for Transplant Immunosuppression
Our lab develops targeted drug delivery strategies to enhance immune regulation in transplantation. By mimicking natural lymphocyte trafficking, we engineered MECA79-coated microparticles to selectively deliver the immunosuppressive drug tacrolimus to lymph nodes. In a heart transplant model, this approach significantly prolonged graft survival while minimizing systemic drug exposure. Our findings highlight the potential of lymph node-targeted therapies to improve transplant immunosuppression, offering a more precise and effective strategy for long-term graft survival.
Azzi, J. et al. Targeted Delivery of Immunomodulators to Lymph Nodes. Cell Reports 15, 1202–1213 (2016). https://doi.org/10.1016/j.celrep.2016.04.007Organ-Targeted Nanotherapy for Enhanced Transplant Immunosuppression
Our lab develops innovative strategies to improve long-term transplant outcomes by reducing early immune activation. We have engineered a nanotechnology-based approach to deliver immunosuppressive agents directly to donor organs before transplantation. By targeting local immune responses within the graft, this ex vivo drug delivery system minimizes systemic exposure while enhancing transplant immunosuppression. Our findings highlight the potential of nanocarrier-based therapies to improve graft survival and reduce chronic immune-mediated injury.
Uehara, M. et al. Nanodelivery of Mycophenolate Mofetil to the Organ Improves Transplant Vasculopathy. ACS Nano 13, 12393–12407 (2019). https://doi.org/10.1021/acsnano.9b05115






